Mixtures differ in how the substances that form them are mixed. Pure substances and mixtures.

Question Video Identifying The Best Description Of A Solution From A Set Of Descriptions Nagwa
The substances in a mixture retain their individual properties.
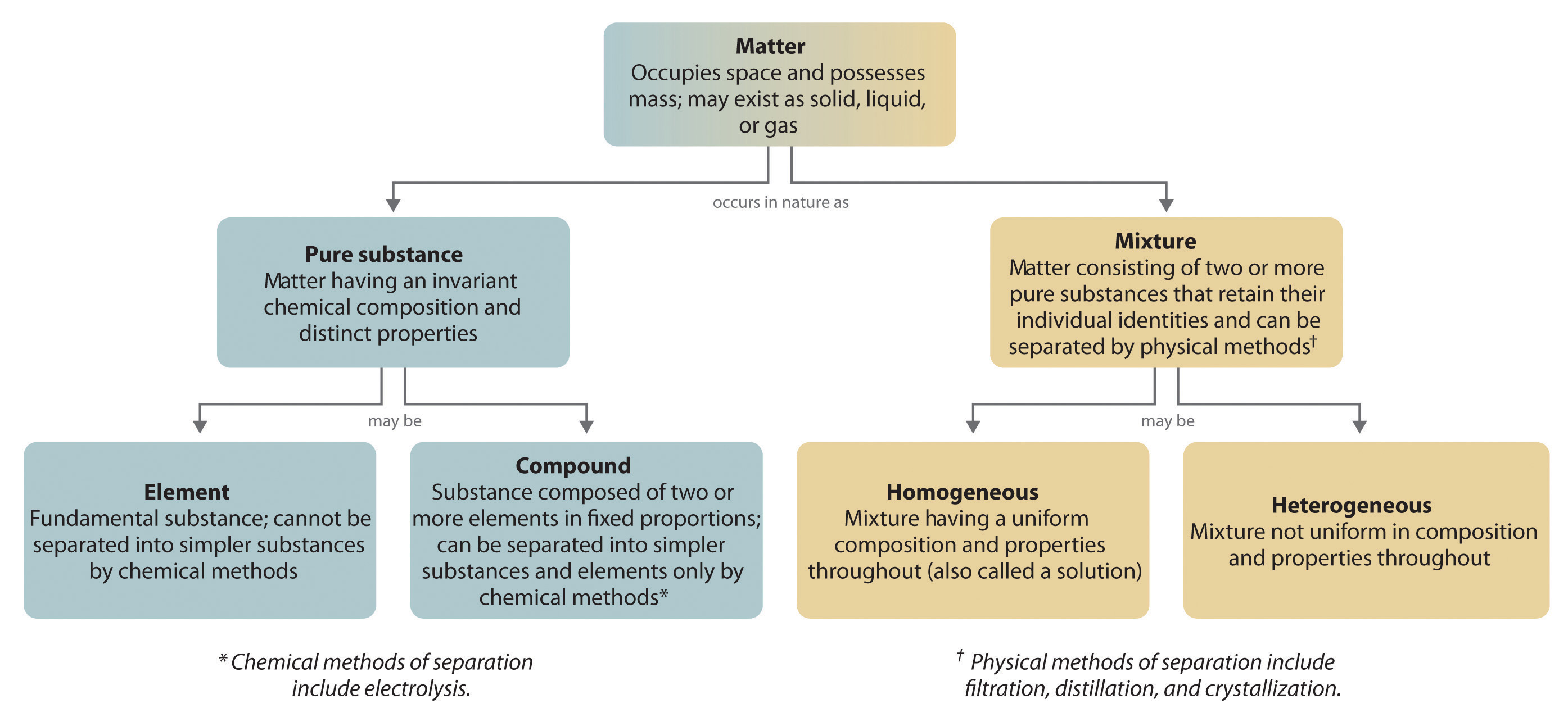
. The properties of NaCl are different from the properties of Na and Cl. Examples of mixtures are. Pure substances are further broken down into elements and compounds.
Pure substances have a definite composition are homogeneous and include elements and compounds. Solutions a special kind of mixture where one substance dissolves in another. We have to separate petroleum into its parts to make it useful.
Mixtures are liquids C. Which of these statements best describes the relationship between matter pure substances and mixtures. Pure substances are further divided into elements and compounds.
How do these differ from mixtures. Pure substances and mixtures. A mixture in which two or more substances are evenly mixed but not bonded together is an mixture also called an.
For example aluminium is an element and ammonia is compound. A mixture of liquids solids or gases can be produced. When sugar is put in water for example it forms a mixture then it.
Substances which have a specific composition and cannot be separated into any constituents are called pure substances. Pure substances can be divided into two groups. Mixtures are composed of several kinds of compounds.
The physical combination of two or more substances is a mixture where the identities of the individual components are retained while the mixture is in form of colloid suspension or solutions. Are substance and mixture same or different. The ability to be compressed.
Comparing and differentiating between substances and mixtures. A pure substance is made up of the same kind of molecules whereas mixture is made up of two different molecules. All compounds have fixed properties ie have a definite chemical composition whereas.
Solutions When substances dissolve and form a homogeneous mixture Suspensions. How is a mixture different from a compound. Heterogeneous Mixtures the parts of the mixture are noticeably different from one another.
Matter can be broken down into two categories. Which is a chemical property. Mixtures can be classified into two types viz.
Substances are composed of pure elements or chemically bonded elements whereas mixtures are composed of non-bonded substances. Photo by Ylanite Koppens from Pexels. Which describes how mixtures differ from substances.
The particles in both a suspension and a colloid can a. 1A pure substance is a form of matter that has a fixed chemical composition and a distinct characteristic while a homogeneous mixture is a mixture of two or more compounds with compositions that are uniform or mixed together in such a way that they are indistinguishable from each other. A mixture is a material composed of two or more simpler substances in chemistry.
Describe the two kinds of pure substances. Up to 24 cash back Petroleum or crude oil is a mixture of different pure substances. A mixture is created when two or more different substances are physically combined and can be separated back into its original substances.
Matter can be divided into two groups. Pure substance is made of only one type of atom or only one type of molecule it has definite and constant composition with distinct chemical properties. There are two types of mixtures.
Each substance in a mixture keeps most of its characteristic properties. Up to 24 cash back Types of Mixtures. 2A pure substance cannot be separated into two.
Mixtures two or more substances that are not chemically combined with each other and can be separated by physical means. Mixtures refer to substances that contain two or more different chemical substances that are not chemically combined together. A bowl of different kinds of candy a pile of different kinds of leaves steel sugar syrup.
Mixtures do not have fixed properties the composition of mixture is variable. Pure substances and mixtures. The substances in the mixture are either homogeneous or heterogeneous in nature.
The two or more substances are existing together despite there being no force acting between them. It comes out of the ground as a thick liquid. Solids liquids and gases can form mixtures.
Homogeneous Mixtures the substances are so evenly distributed that it is difficult to distinguish one substance in the mixture from another. The proportions of the substances that are present in the mixture vary in an indefinite manner. The answer is.
A mixture such as trail mix in which the substances are not evenly mixed is an mixture. Many products that we use in everyday life are made from petroleum. Mixtures are commonly found in nature.
A new substance is formed after the constituents are chemically combined and in this regard a compound will definitely have different properties from its original constituents. Mixtures have two or more components. Heterogeneous and homogeneous mixtures.
The pure substances possess similar properties and composition throughout on the other hand in mixtures properties and composition vary as the constituents are mixed in indefinite proportion. A substance can be considered anything liquid while a mixture is something that has more than one component in it. Mixtures contain only one kind of atom.
Matter can be divided into two groups. Up to 24 cash back By asking these questions scientists can classify matter into. Mixtures and matter are two of many different types of pure substances.
It is uniform in composition and the parts that make up the mixture cannot be separated from one another through physical means It is not uniform in composition and the parts that make up the mixture can be separated from one another through chemical means Question 16 900 seconds Q. The combination of two or more pure substances is called a mixture. These include fuels plastics asphalt many medicines and fertilizers.
Such materials can be compounds or chemical elements. A pure substance contains only one kind of compound. It can be the same molecule or atom.
However its important to look at them individually in order to understand the nature of these substances better. Mixtures are physical combinations of pure substances. Mixtures can be separated physically D.
The main difference between pure substance and mixture lies in their composition. Mixtures are homogeneous B. Mixtures do not have a definite composition and may be homogeneous or heterogeneous.
The Characteristics of the Mixture are as Follows-. So in general they are different.

1 3 Classification Of Matter Chemistry Libretexts

Lesson Explainer Mixtures Nagwa
What Is The Difference Between Mixtures And Substances Quora
0 Comments